FAQ
If you have a question, don’t hesitate to call our main number: 575-622-5600 or 800-753-7284. Dial 0 and one of our client service representatives will answer your questions or transfer you to the appropriate PCNM staff member.
What organisms are included in the Extended Vaginosis Panel?
What does it mean if a ThinPrep Pap test is Unsatisfactory for Evaluation due to presence of lubricant?
How do I contact PCNM’s Marketing Specialist?
Does cytology reported as negative but absent or insufficient EC/TZ component need to be repeated?
What organisms are included in the Extended Vaginosis Panel?
The Extended Vaginosis Panel tests for the following organisms using Real-Time PCR:
- Candida albicans
- Candida tropicalis
- Candida parapsilosis
- Candida glabrata
- Mobiluncus mulieris and M. curtisii
- Gardnerella vaginalis
- Atopobium vaginae
- Bacterial Vaginosis Associated Bacteria 2 (BVAB2)
- Megasphaera species (Type 1 and Type 2)
A separate urogenital Mycoplasma & Ureaplasma panel can also be requested.
What does it mean if a ThinPrep Pap test is Unsatisfactory for Evaluation due to presence of lubricant?The ThinPrep Pap test is prepared using a method that filters the liquid-based specimen through a membrane filter. Carbomer/carbopol polymer thickening agents found in lubricants, spermicides and intravaginal medication delivery systems can cause clogging of the filter membrane reducing the number of cells on the slide and causing clumping of the cells so they can’t be evaluated. See the photomicrographs below which show the difference between an “adequate” ThinPrep Pap preparation and one that has been adversely affected by “lubricant-like” materials. See the ThinPrep Customer Lubricant Letter for more details and collection guidelines.
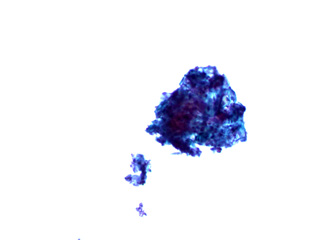
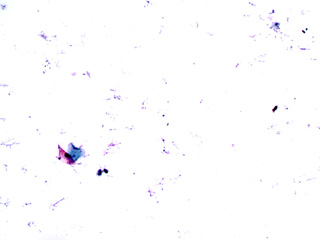
How do I contact PCNM’s Marketing Specialist?
For questions or concerns about service or to schedule a site visit, please contact us at 575-624-4057.
Does cytology reported as negative but absent or insufficient EC/TZ component need to be repeated?
According to the most recent ASCCP guidelines “2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors”
http://www.asccp.org/asccp-guidelines
Box 1: Essential Changes From Prior Management Guidelines*
Cytology reported as negative but lacking endocervical cells can be managed without early repeat.
*Prior management guidelines were from the ‘‘2006 Consensus Guidelines for the Management of Women With Abnormal Cervical Screening Tests’’ (6). Prior guidelines not changed were retained.
“Management of Women With Cytology Reported as Negative but With Absent or Insufficient EC/TZ Component”
“For women aged 21-29 years with negative cytology and absent or insufficient EC/TZ component, routine screening is recommended. HPV testing is unacceptable (BIII). For women aged 30 years and older with cytology reported as negative and with absent or insufficient EC/TZ component and no or unknown HPV test result, HPV testing is preferred (BIII). Repeat cytology in 3 years is acceptable if HPV testing is not performed (BIII). If the HPV test is done and is negative, return to routine screening is recommended (BIII). If the HPV test is positive, repeating both tests in 1 year is acceptable (BIII). Genotyping is also acceptable; if HPV type 16 or type 18 is present, colposcopy is recommended (BII). If HPV type 16 and type 18 are absent, repeat co-testing in 12 >months is recommended (BIII).
See the complete updated guidelines at file:///Users/sfink/Downloads/ASCCP_Cervical_Cancer_Screening_Recommendations.pdf.



